Bleaching Process
Bleaching is an important process of wet processes in the textile industry. It is a pretreatment process of dyeing and printing which removes natural color from the fabric to get a bright white fabric. Bleaching is a process used in textile production to whiten fabrics. The bleaching process is done chemically in dyeing and printing textile mills. So the Bleaching Process is a Chemistry. This process also increases the absorbency capacity of fabrics. After completing the bleaching process, the grey fabric becomes very white and bright. Scouring is the previous process of bleaching in the Textile wet process. Then Mercerizing process only before dyeing. This article is all about the Chemistry of the Bleaching Process in the Textile Industry wet processes. Hope you would love this to read.
Definition of bleaching in Textile
The term bleaching is very common, even general people can realize its meaning. One of the reasons is “Bleaching powder” is used in different industries and for domestic purposes as well for a long period of time. But if we talk about “Textile Bleaching”, it has a definite definition and it is also a common vital method in industrial practice. So simply Bleaching expresses a “Color (Natural and Applied) removal process from textile substrates (fabric or garments), to obtain a pure and permanent whiteness”. It can be done by reducing or oxidizing but in the case of reducing methods color can be back to the original color by further oxidization.
The bleaching process varies according to Bleaching Chemicals, Applied Substrates, Application Medium, and Methods.
Objectives of the Bleaching Process Chemistry in Textile
Here are some major reasons but it has a few others appeal as well
- Remove the natural raw hue of the grey fabric.
- To make a permanent white effect.
- Make the textile fabric bright and white.
- Increasing the absorbency power and dye affinity of textile fabric.
- Prepare for fabric for the dyeing process.
- To obtain an even hue in the final product.
- Better Dyeing and Printing outputs.

Common Bleaching Agents:
- Sodium Hypochlorite (NaOCl)
- Sodium Chlorite (NaClO2)
- Hydrogen per Oxide (H2O2)
Among them, NaOCl is the cheapest and Strongest. It was invented in 1820. But in recent eras, H2O2 is mostly used and it is called a universal Bleaching Agent.
Preparation of Sodium Hypochlorite (NaOCl) : 2NaOH + Cl2→NaCl + NaOCl +H2O [Less than 27℃, instant preparation]
- Reaction: Redox potential of NaOCl is (1400-1550), here “O” is responsible for Bleaching.
- NaOCl +H2O→NaOH + HOCl [PH 11 to 11.5]
- At lower PH: HOCl→HCl + [O]
If we add more acid reaction moves to the left and NaOH is neutralized and the Maximum HOCl yield is at PH 6 to 6.5. In this Ph range maximum “O” is generated. So very fast Bleaching.
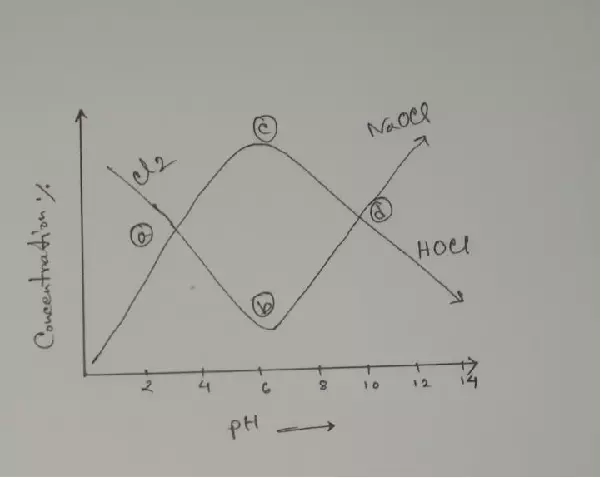
At neutral PH (c & d in figure) maximum HOCl concentration and Bleaching take a few minutes. So, it is tough to control and risk of materials damage. So, in general, Bleaching is carried out in PH 9 to 11.
Typical Recipe-
- NaOCl – 3 to 5 gm/L
- PH – 9 to 11
- Temp. – 30℃
- Time (J-Box m/c) – 30 min in PH 9 (Used for the continuous process)
- 4 hours at PH 11 (Used for the batch process)
- If NaOCl concentration > 8% then after 30 minutes, Cotton starts degradation. (-OH) of 1, 4, 2 in Cellulose ring converted into (-CHO and -CO-). In presence of an Alkali, it starts a reaction in (1, 4 points).
- As the whole process is carried out at room temperature so it is energy-saving.
- Also, it needs less NaOCl, as it is a strong agent.
Anti-chloro treatment: In storage in generates yellow in the substrate for Chloramine. Chlorine is not Biodegradable. So, we need to remove residual chlorine with 1% Sodium Sulphite.
** NaClO2 (Next Bleaching Agent):
- Here Bleaching is done under acidic pH.
- Redox potential 1040-1200
- It is a mild agent (yellow in color and marketed in powder form)
- Used for generally polyester.
- Less used for Cotton fabric
- [O] is responsible for Bleaching Action
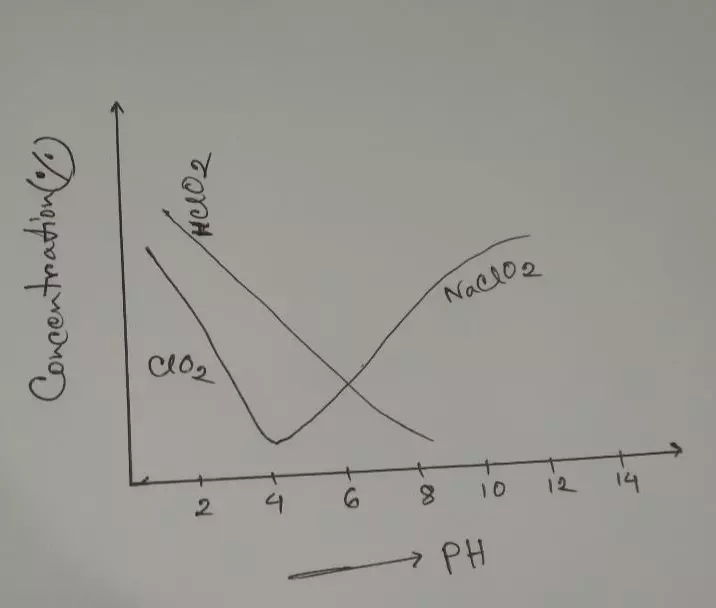
- Reaction: NaClO2 + H2O→NaOH +HClO2 [PH:8.5]
- HClO2→HCl +2[O]
- Recipe:
- NaClO2 – 1-2% (according to materials weight)
- Mild Acid – adjustable (to maintain PH 3-4)
- Time – 1-2 hours
- Temp. – 90 to 100℃
- ***NaNO2 is used for corrosion resistance. ***At PH (3-4) maximum Bleaching action.
H2O2 is the most important and most commonly used in industry at present. It was first introduced in 1888 but from 1930 it was used on a commercial scale. The major advantages of H2O2 are:
• No yellow effect
• Environment friendly
• No byproduct
In past, it was considered that according to the given reaction below O2 is responsible for Bleaching but actually HO2- is responsible for Bleaching.
- 2 H2O2→2H2O+ O2
- Reactions: H2O2→ H+ + HO2– – (Acidic in Nature)
But if we add acid reaction moves left direction: H2O2 + acid→H+ + HO2–
- In addition to the alkali reaction moves forward directions: H2O2 + OH–→ H2O + HO2–
- HO2– + Color → -OH– + Oxidized color
So HO2– (peroxyl) is responsible for Bleaching. But to generate HO2–
We need to add alkali. So, that’s why Alkali is called an activator. Some common activators are NaOH, Na2CO3, Na3PO4, TSP, and NH3. Activator selections depend on fabrics.
PH: For H2O2 bleaching PH is one of the crucial factors, in general PH 10.5 is mostly used for Cotton. Above PH 10.5, bleaching is very fast, and below PH 10.5, takes a long time to finish Bleaching. But for Silk and Wool PH range is 8-9.
Stabilizer: The purpose of using a stabilizer is to provide a prolonged time of peroxyl ion (HO2). Salicylic Acid and Sodium Silicate are used for Stabilizer. Salicylic Acid acts as a gel and is entrapped (HO2–). Increase its lifetime. Besides Sodium Silicate is Highly viscose and deposited in hard water and blocks the instruments.
H2O2 in the market is available in several concentrations. 50% concentration (Used in industry, Transportation factors), 35%, and 30% (Used in Lab). ** H2O2 is very sensitive to metal ions. In presence of Fe, and Cu it gets decomposed. So, both water and fabric must be free from any kind of metal ion. **Sometimes it has seen fine holes all over the fabric. The reason for it is “Rusting iron bar over the Bleaching batch. So, it must be covered with polyethylene. Or used sequestering or Metal chelating (EDTA) Agent”.
Recipe:
- H2O2 (50% conc.): 1-2% (OWF)
- NaOH (Activator) : Adjustable (to maintain PH 10.5) Sodium Silicate (Stabilizer) : 1-2%
- Non-ionic Surfactant : 3 gm/L EDTA (Metal Chelating Agent): 0.01%
- Time: varies according to Temperature. (16 Hours at 30℃ and 1 minutes at 100℃) Most preferable for 95℃ and time 2 hours.
** After H2O2 Bleaching need an Acetic Acid wash to neutralize residual Alkali. ** But still now to improve whiteness and brightness we need to use UBA or OBA.
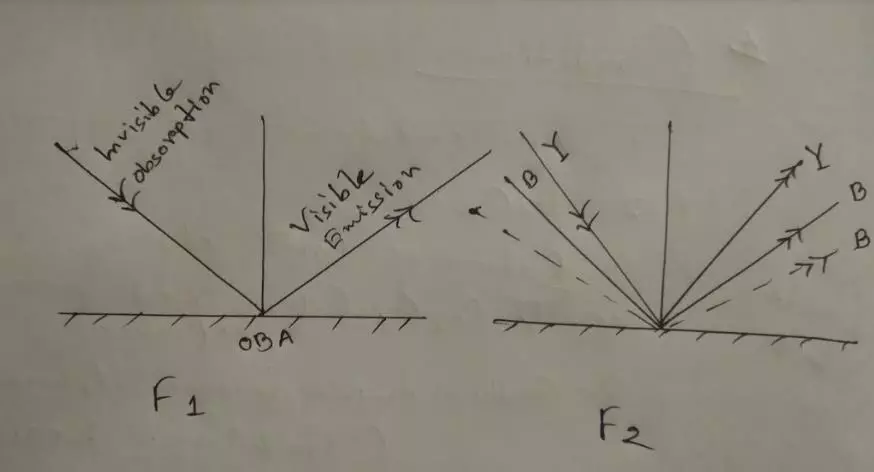
Mechanism of OBA: OBA absorbed the invisible region but reflect in the visible region. So resultant reflection (Y= B+B) makes more Bright, Blueish, and White appearance. Here is the figure:
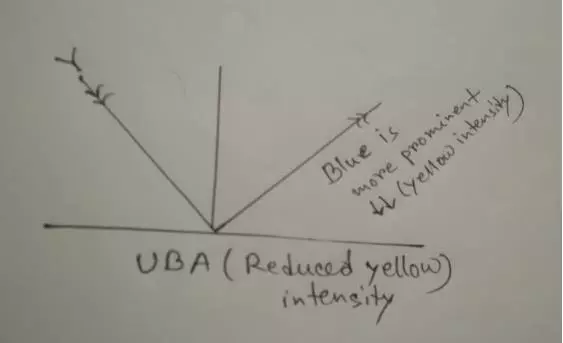
Mechanism of UBA:
It absorbed a yellow portion of natural light. So, the intensity of the yellow portion reduces. That’s why the blue portion seems brighter and the fabric has appeared more Blueish, Bright, and white appearance. Here is the figure:
Hope this writing gives us the concept of Bleaching Chemistry in the Textile Industry. *** So here is a brief about Bleaching Chemistry, I am thankful to all of my teachers***
- Author: Najmul Hossain
- Lecturer, Department of Textile Engineering
- Chittagong National Engineering College, Affiliated with the University of Chittagong
- LinkedIn: Najmul Hossain
